Lab Planning & Design
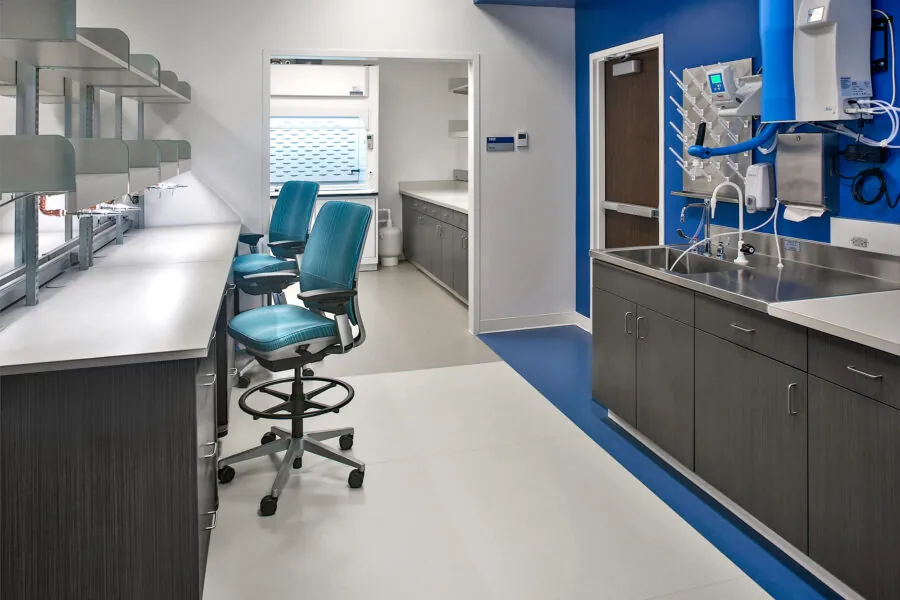
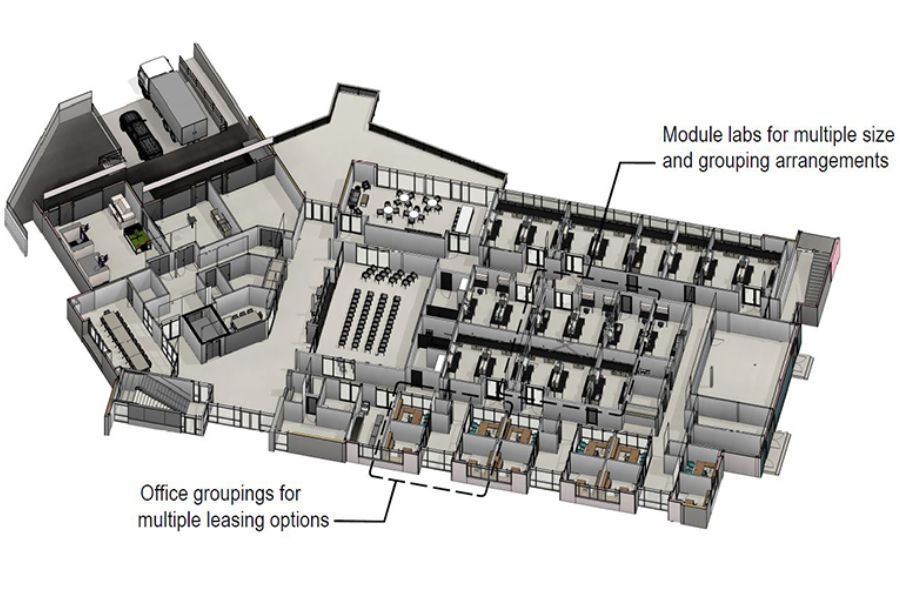
We develop effective, efficient, and creative laboratory environments that enable science to advance in a cost-sensitive corporate and academic world. Our specialized laboratory and vivarium planners, programmers, and designers have developed research, development, and production facilities for public and private clients. These range from academic research and teaching institutions to governmental agencies and private companies, each requiring tailored solutions to meet their unique needs and goals.
View Projects